If we weigh an element equal to its atomic mass in grams,then it contains 6.022×1023 atoms of the element.The gram atomic mass of the element as well as 6.022×1023 atoms of the element, both represent 1 mole of element.1 mole of any species (atoms,molecules,ions) is equal to its atomic mass or molecular mass in grams.
No. of particles(atoms, molecules, ions) in 1 mole=6.022×1023
This number is called as AVOGADRO’S CONSTANT
1 mole of atom=6.022×1023atoms
1 mole of molecules=6.022×1023molecules
Mole of atoms
1 mole of atoms of an element has a mass equal to the gram atomic mass of the element.
For Ex: 1 mole of oxygen atoms=16 grams
The symbol of an element represents 1 mole of atoms of that element.
o represents 1 mole of oxygen atoms.
2 o represents 2 moles of oxygen atoms.
Mole of Molecule
1 mole of molecules of a substance has a mass equal to the gram molecular mass of the substance.
For Ex:The gram molecular mass of oxygen is 32 grams.O2 represents 1 mole of oxygen molecule
2 O2 represents 2 mole of oxygen molecule
1 mole of molecule=2u=2g=6.022×1023 molecule
1 mole of water molecule=18u=18g=6.022×1023 water molecule
1 mole of sodium atom=23u=23g=6.022×1023 sodium atom
2)The amount of a substance equal to its gram atomic mass or molecular mass.
3)A definite number of atoms,molecules or ions of a substance.

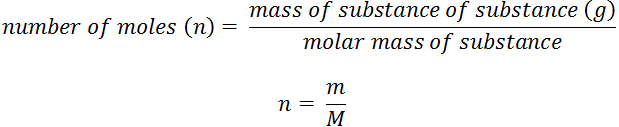
Nice
Thanks
wonderful…..ba!
Thanks
It’s not 6.022 x 1023 , it is 6.022 x 10 to the power 23 ..pls its my request to not publish wrong information …plss .
Thank u so much for pointing out the error.It was formatting error in word press.I have published the correct information only.
I can’t understand the mole concept but by reading this I got the idea about the molar concept. Thank u.
Yes u r right
Thank u arpan.
Very helpful thanks
V gud
Wow, it’s wonderful… I learnt this topic of moles in a very easy way because of you…
Very nice explanation
Thank you so much
Thanks for the notes
It helped me to again remember the formulas of the topic
thanks a lot this has helped with the concept about mole concept
Nice………
it was really helpful for me to study during exam.
i was actually struggling to learn and by heart this mole concept.
but after referring to your notes i understood this concept crystal clear.
i helped my friends by sharing this website link too.
and we are thankful to you madam.
thank you ma’am .
Good, Children can easily understand
Really it is from my heart
Your The Best
Keep it up
Good and Thanks Mam
Thanks Mam
You are the Best
Thanks for the help.
i understood mole concept very nicely by reading this……
thanks a lot shilpa mam………..
Thanks mam .. mole concept is clear now
Thank you sooo much…