Question 1 What is a fuel? Give few examples?
Question 2 What are the characteristics of an ideal fuel or good fuel?
Question 3 What is a source of energy?
Question 4 State four characteristics of a good source of energy?
Question 5 What is meant by renewable sources of energy? Give few examples?
Question 6 What is meant by non- renewable sources of energy? Give few examples?
Question 7 Why are fossil fuels classified as non-renewable sources of energy?
Question 8 Why coal is considered as a non-renewable source whereas wood is a renewable source ?
Question 9 Define ignition temperature of a fuel?
Question 10 What is meant by calorific value of a fuel?
Contents
Sources of Energy
A source of energy is one which can provide adequate amount of energy in a convenient form over a long period of time. All the sources of energy can be divided into two main categories:
Non-renewable sources of energy and Renewable sources of energy.
(1) Non-Renewable Sources of Energy
Those sources of energy which have accumulated in nature over a very, very long time and cannot be quickly replaced when exhausted are called non-renewable sources of energy.
For example: Coal is a non-renewable source of energy because coal has accumulated in the earth over a very, very long time, and if all the coat gets exhausted, it cannot be produced quickly in nature.
The non-renewable sources of energy are: Fossil fuels (Coal, Petroleum and Natural gas), and Nuclear fuels (such as Uranium). Non-renewable sources of energy are dug out from the earth.
The non-renewable sources of energy are also called conventional sources of energy. The non-renewable sources of energy like fossil fuels (coal, petroleum and natural gas) are present in a limited amount in the earth. Once exhausted, they will not be available to us again.
Since the non-renewable sources of energy can get exhausted one day, they are also known as exhaustible sources of energy.
(2) Renewable Sources of Energy
Those sources of energy which are being produced continuously in nature and are inexhaustible, are called renewable sources of energy.
For example: Wood is a renewable source of energy because if trees are cut from the forests for obtaining wood, then more trees will grow in the forest in due course of time. So, the loss of wood by cutting trees is made good by nature.
The renewable sources of energy are Hydro energy (Energy from flowing water), Wind energy, Solar energy, Energy from sea (Tidal energy, Sea-wave energy and Ocean thermal energy), Geothermal energy, Biomass energy (Energy from bio-fuels such as Wood, Biogas and Alcohol), and Hydrogen.
The renewable sources of energy are also called non-conventional sources of energy. These sources of energy can be used again and again endlessly They will never get exhausted. Since renewable sources of energy will never get exhausted, so they are also known as inexhaustible sources of energy.
Good Source of Energy
Whenever work has to be done, energy is needed. This energy is supplied by a source of energy. Different sources of energy are used depending on the type of work to be done.
A good source of energy is one:
(1) which would do a large amount of work per unit mass (or per unit volume),
(2) which is cheap and easily available,
(3) which is easy to store and transport,
(4) which is safe to handle and use, and
(5) which does not cause environmental pollution.
The most common sources of energy available to us are the fuels.
Fuels
The materials which are burnt to produce heat energy are known as fuels.
Examples of fuels are: Diesel and Petrol.
Fuels are the concentrated store-house of energy. This energy is released in the form of heat when the fuels are burnt. Different fuels produce different amounts of heat on burning. Some fuels produce more heat whereas others produce less heat.
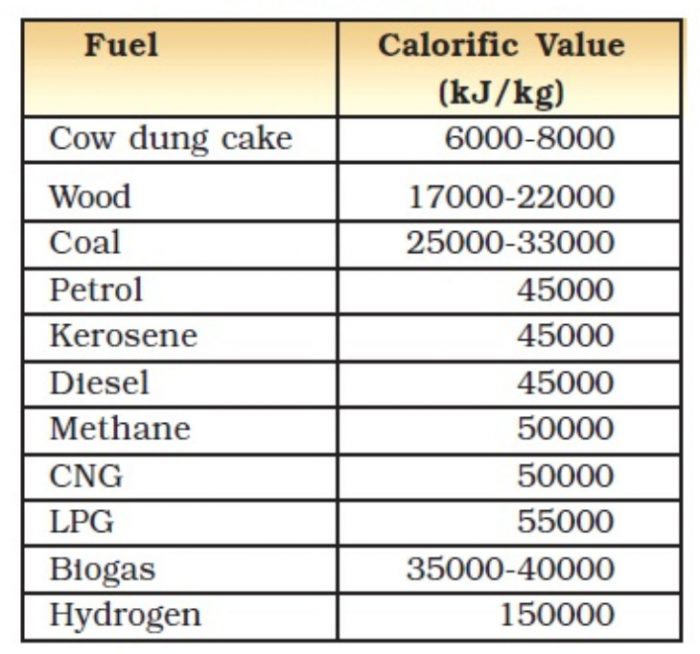
The usefulness of a fuel is measured in terms of its calorific value. Higher the calorific value, better the fuel will be.
The amount of heat produced by burning a unit mass of the fuel completely is known as its calorific value. The unit of mass usually taken for measuring the calorific value of a fuel is “gram”. The amount of heat produced by burning 1 gram of a fuel completely is called its calorific value.
For example : When one gram of a carbon fuel (like charcoal) is burned completely, it produces about 33000 joules of heat, so the calorific value of charcoal is 33000 joules per gram or 33000 /g.
Since joule is a very small unit of heat energy, so the calorific value is usually expressed as kilojoules per gram (kJ/g). Thus, the calorific value of charcoal becomes 33 kilojoules per gram which is written in short form as 33 k/g.
Thus, the common unit of measuring calorific value is kilojoules per gram (kJ/g). The calorific value of a fuel represents the heat value of the fuel.
The calorific value of kerosene oil is 45 kJ/g. The calorific value of kerosene oil is 45 kilojoules per gram, we mean that if 1 gram of kerosene oil is burnt completely, then it will produce 45 kilojoules of heat energy.
Hydrogen gas has the highest calorific value of 150 kilojoules per gram Thus, because of its high calorific value, hydrogen is an extremely good fuel.
Most of the common fuels are the compounds of hydrogen and carbon called ‘hydrocarbons’. Since hydrogen has the highest value, therefore, a fuel containing higher percentage of hydrogen will have a higher calorific value than another fuel which has a lower percentage of hydrogen in it.
For example, LPG has a higher percentage of hydrogen than coal, so LPG has a higher calorific value than coal.
Before a fuel can catch fire and burn, it must be heated to a certain minimum temperature. The minimum temperature to which a fuel must be heated so that it may catch fire and start burning, is known as its ignition temperature. When we apply a burning matchstick (or a lighter spark) to the burner of a gas stove, we actually supply a little heat to cooking gas coming out of gas burner so that it gets heated to its ignition temperature and start burning. No fuel can burn unless it is heated to its ignition temperature.
Characteristics of an Ideal Fuel (or Good Fuel)
(1) It should have a high calorific value: An ideal fuel (or good fuel) is that which gives us more heat per unit mass.
(2) It should burn without giving out any smoke or harmful gases: An ideal fuel (or good fuel) is that which does not pollute air on burning by giving out smoke or poisonous gases.
(3) It should have a proper ignition temperature, so that it can be burned easily: The ignition temperature of an ideal fuel (or good fuel) should neither be too low nor too high. Because if the ignition temperature of the fuel is very low, then the fuel will catch fire too easily and hence it will be very unsafe to use it. If the ignition temperature is too high, then it will be very difficult to light the fuel.
(4) It should be cheap and easily available: An ideal fuel (or good fuel) is that which is not expensive and which is available in plenty everywhere.
(5) It should be easy to handle, safe to transport, and convenient to store: An ideal fuel (or good fuel) is that which does not create any safety risks during handling, during its transportation from one place to another or during its storage.
(6) It should not leave much ash behind after burning: An ideal fuel (or good fuel) should have low percentage of non-volatile materials which do not burn, so that it may burn completely without leaving much ash.
(7) It should burn smoothly: An ideal fuel (or good fuel) should have a moderate rate of combustion, and burn at a steady rate. The fuel should not burn either too fast or too slow.
Suppose we have two fuels A and B. The calorific value of fuel A is 55 kJ/g and its ignition temperature is 20°C whereas the calorific value of fuel B is 50 kJ/g and its ignition temperature is 80°C Now, we find that fuel A has a higher calorific value of 55 KJ/g than that of fuel B. So, at first sight it would appear that fuel A is a better fuel than fuel B. But it is not so. This is because fuel A has a very low ignition temperature of 20°C due to which it can catch fire easily and hence it is unsafe to use. On the other hand, though the calorific value of fuel B is little less (than that of fuel A), but it has the right ignition temperature of 80°C which is neither very low nor very high. So, here fuel B will be a better fuel on the basis of its appropriate ignition temperature.
On burning, fuel A produces gases like CO2, H2O, CO, SO2, SO3 etc. whereas fuel B produces only CO2 and H2O. Out of these gases carbon monoxide (CO), sulphur dioxide (SO2) and sulphur trioxide (SO3) are poisonous gases or harmful gases. Now, since fuel A produces poisonous gases like CO, SO2, and SO3, on burning, therefore, fuel A is not a good fuel. Burning of fuel B produces only harmless products like carbon dioxide (CO2) and water vapour (H2O), so fuel B is a better fuel.
If a fuel burns with an explosion, it will not be a good fuel and if it leaves behind a lot of ash on burning, even then it cannot be a good fuel.
Wow! The language is so simple, I clearly understand everything…thanks for creating this website!
Amazing notes wow wow
Thank you for these notes, it is clear and understandable