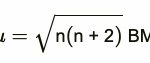Potassium Dichromate Properties of Potassium Dichromate 1) Colour and melting point : It is an orange red crystalline solid having melting point of 670 K. 2) Solubility : It is moderately soluble in cold water but readily soluble in hot water. 3) Action of heat : It decomposes on heating to form potassium chromate , chromic acid and oxide. 4 K2Cr2O7 → 4K2CrO4 + 2 … [Read more...] about Potassium Dichromate
Class 12
Trends in Stability of Higher Oxidation States and Magnetic Properties
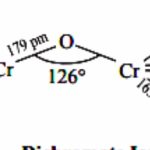
Trends in Stability of Higher Oxidation States Standard electrode potential data provide valuable information about the stabilities of different oxidation states shown by an element. The highest oxidation states are shown generally among halides and oxides. 1) In metal halides The transition elements react with halogens at high temperatures to form transition metal … [Read more...] about Trends in Stability of Higher Oxidation States and Magnetic Properties
Tendency to form Complexes, Formation of Interstitial compounds and Alloys, Catalytic Properties
Tendency to form Complexes The transition elements form a large number of coordination complexes. The transition metal ions bind to a number of anions or neutral molecules in these complexes. The common examples are [Ni(NH3)6]3+, [Co(NH3)6]3+ , [Fe(CN)6]3+ , [Fe(CN)6]4-, [Cu(NH3)4]2+. The high tendency of transition metal ions to form complexes is due too : 1) … [Read more...] about Tendency to form Complexes, Formation of Interstitial compounds and Alloys, Catalytic Properties
Standard Electrode Potential and Formation of Coloured Ions
Standard Electrode Potentials The magnitude of ionization enthalpy gives the amount of energy required to remove electrons to form a particular oxidation state of the metal in a compound. Thus, the value of ionisation enthalpies gives information regarding the thermodynamic stability of the transition metal compounds in different oxidation states. Smaller the ionisation … [Read more...] about Standard Electrode Potential and Formation of Coloured Ions
Melting & Boiling Point, Ionisation Enthalpies & Oxidation State of Transition Elements
Melting and Boiling Points The transition metals have very high melting and boiling points. The melting points of these metals rise to a maximum value and then decrease with increase in atomic number. However, manganese and technetium metals have abnormally low melting points. Explanation: The high melting and boiling points of these metals are due to strong metallic … [Read more...] about Melting & Boiling Point, Ionisation Enthalpies & Oxidation State of Transition Elements