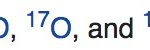Oxoacids of Sulphur Sulphuric Acid Manufacture of Sulphuric Acid Sulphuric acid can be manufactured by Contact process. The process involves the following steps: (i) Preparation of sulphur dioxide Sulphur dioxide is prepared by burning sulphur or iron pyrites in excess of air. S+O2 → SO2 4FeS2 + 11O2 → 2Fe2O3 + 8SO2 Iron pyrites (ii) Oxidation of … [Read more...] about Sulphuric Acid
Chemistry
Allotropes of Sulphur and Sulphur Dioxide
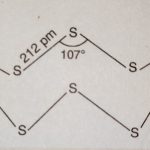
Sulphur occurs in the free state as well as in the combined state. Allotropes of Sulphur Sulphur exists in numerous allotropic forms of which three forms are the most important. These three main allotropic forms are: (i) Rhombic sulphur (iii) Plastic sulphur (ii) Monoclinic sulphur (i) Rhombic sulphur or α-Sulphur (i) It is the common … [Read more...] about Allotropes of Sulphur and Sulphur Dioxide
Ozone
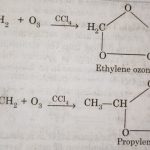
Ozone Ozone is an allotropic form of oxygen. It is present in the upper atmosphere (about 20 km above the surface of the earth). It is believed to be formed in the upper atmosphere by the action of ultraviolet rays on oxygen as 3O2 + Ultraviolet rays → 2O3 ΔH298K = 142.7 kJ mol-1 Ultraviolet rays, which are harmful to human beings, are absorbed by oxygen to form … [Read more...] about Ozone
Dioxygen
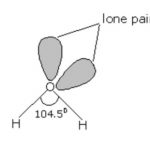
Dioxygen It occurs in three isotopic forms such as in molecular state, it exists as O2 and is called dioxygen or simply oxygen. All the dioxygen in the atmosphere is believed to be due to the photosynthesis taking place in green plants in the presence of sunlight. H2O + x CO2 → (CH2O)x + xO2 Sunlight … [Read more...] about Dioxygen
Chemical Properties of Group 16 Elements
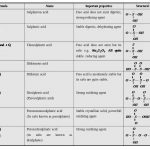
Chemical Properties of Group 16 Oxygen shows anomalous behaviour. This is due to its small size, high electronegativity, high ionisation enthalpy and absence of d-orbitals in its valence shell. Due to the absence of d-orbitals in oxygen, its covalency is limited to four. The valence shell can be expanded using vacant d-orbitals and hence covalence exceeds four. Both … [Read more...] about Chemical Properties of Group 16 Elements