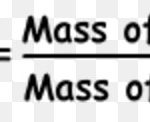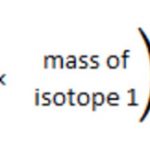A solution is defined as a homogeneous mixture of two or more chemically non reacting substances, the relative amount of which can be varied up to a certain limit. If a solution consist of only two components it is called a binary solution. The component present in smaller amount is called solute while the other present in larger amount is called solvent The … [Read more...] about Mole Concept in Solutions
Some Basic Concepts of Chemistry
Atomic and Molecular mass
As an atom is so small a particle that it cannot be seen or isolated ,therefore it is impossible to determine the actual mass of a single atom by weighing it. The problem was finally solved by Avogadro's hypothesis. If equal volumes of two different gases are taken under similar conditions of temperature and pressure and then weighted, the ratio of their masses will be … [Read more...] about Atomic and Molecular mass
Avogadro’s Hypothesis
Berzelius a Swedish chemist, gave a hypothesis called Berzelius hypothesis which states that: Equal volume of all gases under similar conditions of temperature and pressure contain equal number of atoms. For Example: Hydrogen + Chlorine → 2 Hydrogen chloride gas 1 vol 1 vol 2 vol n atoms n atoms … [Read more...] about Avogadro’s Hypothesis
Dalton’s Atomic Theory
Dalton's Atomic Theory John Dalton in 1808 put forward theory known as Dalton's atomic theory The main points of this theory are: (1) Matter is made up of extremely small indivisible particles called atoms. (2) Atoms of the same element are identical in all respect i.e. shape, size and mass. (3) Atoms of different elements have different masses, sizes and also … [Read more...] about Dalton’s Atomic Theory
Laws of Chemical Combination
Law of Conservation of Mass This Law was studied by French chemist Antoine lavoisier in 1789 This law states that In all physical and chemical changes, the total mass of the reactants is equal to that of the products. Or Mass can neither be created nor destroyed. This law is also called as the law of indestructibility of matter. The mass and energy are … [Read more...] about Laws of Chemical Combination