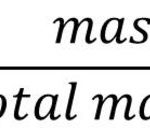The reactant which reacts completely in the reaction is called limiting reactant or limiting reagent. The reactant which is not consumed completely in the reaction is called excess reactant . Question : 3 g of H2 react with 29 g of O2 to form H20.Which is the limiting reagent ? Answer: Thus O2 is present in excess.Hence H2 is the limiting reagent. … [Read more...] about Limiting Reagent
Some Basic Concepts of Chemistry
Balancing Of A Chemical Equation
Balancing of a chemical equation means making the number of atoms of each element equal on both sides of the equation. The methods of balancing equation are: 1) Hit and Trial Method: The simplest method to balance a chemical equation is by hit and trial method. Step 1 : Write down the correct formula of the reactants and products with plus sign in between with an … [Read more...] about Balancing Of A Chemical Equation
Empirical and Molecular Formula
Calculation of Percentage Composition The percentage of any element or constituent in a compound is the number of parts by mass of that element or constituent present in 100 parts by mass of the compound. Step 1 : Calculate the molecular mass of the compound from its formula by adding the atomic masses of the element present. Step 2 : Calculate the … [Read more...] about Empirical and Molecular Formula
Dimensional Analysis
Dimensional Analysis Any calculations involving the use of the dimensions of the different physical quantities involved is called dimensional analysis. It is used for any one of the following purpose: (1) To convert a physical quantity in one type of units into some other units:The method used is called factor label method or unit factor method. (a) First determine … [Read more...] about Dimensional Analysis
Mole Concept
Mole Concept Some Basic Concepts of Chemistry Class 11 Avogadro's number or Avogadro's constant (NA) One gram atom of any element contains the same number of atoms and one gram molecule of any substance contains the same number of molecules. The value was found to be 6.022137 × 1023 The value generally used is 6.022 × 1023 . This is called Avogadro's number … [Read more...] about Mole Concept