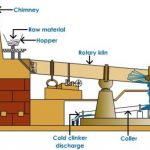
Cement is essentially a finely powdered mixture of calcium silicate and aluminates along with small quantities of gypsum which sets into a hard stone like mass when treated with water. Average composition of Portland cement Cement is obtained by combining a material rich in lime, CaO with other materials such as clay, which contains silica, SiO2 along with oxides of … [Read more...] about Cement
Chemistry
Anomalous Behaviour of Beryllium
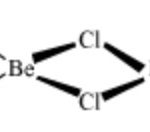
Anomalous Behaviour of Beryllium Beryllium , the first member of alkaline earth metals, show an anomalous behaviour , i.e. differ from the rest of the members of its family. The main reason for this difference are as follow: 1) Exceptionally small atomic and ionic size. 2) High ionisation enthalpy 3) Absence of d-orbital in its valence shell Some important … [Read more...] about Anomalous Behaviour of Beryllium
Characteristics Of Compounds Of Alkaline Earth Metals
Due to higher enthalpy of formation in the solid state and due to higher hydration enthalpy in the aqueous solution, alkaline earth metals uniformally form bipositive ions. Due to increased nuclear charge and smaller size, alkaline earth metals form compounds which are less ionic than the corresponding compounds of the alkali metals. Oxides and Hydroxides Oxides The … [Read more...] about Characteristics Of Compounds Of Alkaline Earth Metals
Reactivity And Electrode Potential Of Alkaline Earth Metals
All the alkaline earth metals are highly reactive elements since they have a strong tendency to lose the two valence s-electrons to form the corresponding dipositive ions having inert gas configuration. Reactivity arises due to their low ionization enthalpies and high negative values of their standard electrode potential. The chemical reactivity of alkaline earth metals … [Read more...] about Reactivity And Electrode Potential Of Alkaline Earth Metals
Physical Properties of Alkaline Earth Metals
The group 2 of the periodic table consist of 6 elements .These are Beryllium (Be), magnesium(Mg), Calcium (Ca), strontium(Sr), barium(Ba), radium (Ra). The name alkaline earth was given since the oxides are alkaline in nature and remain unaffected by heat or fire and exist in Earth's crust. Alkaline earth metals are also highly reactive and hence do not occur in the free … [Read more...] about Physical Properties of Alkaline Earth Metals