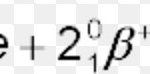Hydrides Dihydrogen combines with a number of elements to form binary compounds called hydrides. The general formula being MHx where M represents the element and x is the number of hydrogen atoms. The various elements which form hydrides are : 1) All main group elements except those of noble gases and probably Indium and thallium. 2)All lanthanides and … [Read more...] about Hydrides
Hydrogen
Preparation of Hydrogen
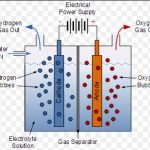
Preparation of Dihydrogen (1) From water Dihydrogen may be obtained from water by reduction either with active metals or by electricity. (a) By the action of water on Active Metals (1) Cold Water : Very active metals i.e. alkali and certain alkaline earth metals like Na, K , Ca react with water at room temperature evolving dihydrogen. 2Na + 2H2O -----------> 2NaOH + … [Read more...] about Preparation of Hydrogen
Occurrence of Hydrogen
Hydrogen is the most abundant element in the universe. The giant planets such as Jupiter and Saturn contain mostly hydrogen. Hydrogen constitutes about half of the mass of Sun and stars. The extremely high temperature of the Sun brings about fusion of hydrogen atoms liberating large amount of energy It is the third most abundant element on the surface of the … [Read more...] about Occurrence of Hydrogen
Position of Hydrogen
1) Hydrogen is the first element in the periodic table. 2) It is the lightest element known. 3) It atomic form exists only at high temperature. 4) It exist as a diatomic molecule i.e. H2 .That is why it is also called dihydrogen. 5) It was discovered by Henry Cavendish in 1766. 6) Its electronic configuration is 1s1 Unique position of Hydrogen in periodic … [Read more...] about Position of Hydrogen