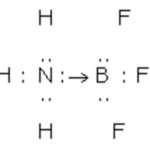In case of certain molecules, a single Lewis structure cannot explain all the properties of the molecule. The molecule is then supposed to have many structures, each of which can explain most of the properties of the molecules but none can explain all the properties of the molecule. The actual structure is in between of all these constituting structure and is called … [Read more...] about Resonance
Chemical Bonding and Molecular Structure
Coordinate Bond
Certain atoms which have complete octets can donate their valence electrons which are not involved in bond formation, to other atoms, which are short of electron. These donated electrons are, therefore, lone pair of electrons and are shared by both the atoms. When in the formation of a bond, the electron pair is donated by one atom but shared by both the atoms so as to … [Read more...] about Coordinate Bond
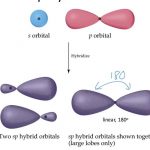
Hybridisation
Hybridisation is defined as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new orbitals of equal energies and identical shape. The new orbitals thus formed are known as hybrid orbitals. Important points about hybridisation … [Read more...] about Hybridisation
Fajan’s Rules
When a cation approaches an anion, the electron cloud of the anion is attracted towards a cation and hence gets distorted. The effect is called polarisation of the anion. The power of cation to polarise anion is called its polarising power and tendency of the anion to get polarised is called polarisability. The greater is the polarisation produced, more is the neutralisation … [Read more...] about Fajan’s Rules
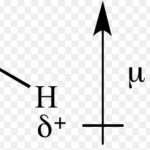
Dipole Moments
In a polar molecule, there are two poles present in the molecule. Hence, the molecule is said to possess an electric dipole. Since the molecule as a whole is electrically neutral ,the negative charges is always equal in magnitude to the positive charge. The product of magnitude of negative or positive charge(q) and the distance between the centres of the positive and … [Read more...] about Dipole Moments