Question 1 Define the term valency?
Question 2 What are monovalent ion. Give example?
Question 3 What are divalent ion. Give example?
Question 4 What are trivalent ion. Give example?
Question 5 What do you mean by chemical formulae of a compound?
Question 6 What are the steps to write chemical formulae of a compound?
Question 7 What is gram atomic mass?
Valency of Ions
The valency of an ion is equal to the charge on the ion.
If an ion has 1 unit charge, its valency is 1 and is called monovalent ion.
For Example : Hydrogen ion, Lithium ion, Potassium ion, ammonium ion, Hydride ion, Fluoride ion, Chloride ion, Bromide ion, hydroxide ion etc.
If an ion has 2 unit of charge, its valency is 2 and is called divalent ion.
For Example : magnesium ion, calcium ion, zinc ion, oxide ion, culphide ion, carbonate ion, sulphate ion etc.
If an ion has 3 unit of charge, its valency is 3 and is called trivalent ion.
For Example : Aluminium ion, Iron ion, nitride ion, phosphide ion, phosphate ion etc.
Chemical Formula
The chemical formula of a compound represents the composition of a molecule of compound in terms of symbols of the elements present in it.
The formula of a compound tells us the kind of atoms as well as the number of atoms of various elements present in one molecule of a compound.
Writing of Chemical Formula
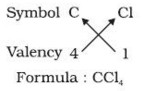
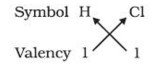
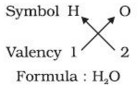
1) In a chemical formula of a compound, the elements present are represented by their symbols and the number of atoms of each element are indicated by writing the digits 2,3,4 etc as subscripts on the right hand side bottom of the symbol.
2) If we know the valencies of elements, then we can work out the formula of their compounds by balancing the valencies of different atoms which occur in compound.
3) First we write the symbol of elements which form compound.
4) Below the symbols of each element,write down the valency.
5) Cross over the valencies of combining atoms.
For Example :
Gram Atomic Mass
The atomic mass of a substance expressed in grams is called as gram atomic mass of that substance.
To write the gram atomic mass of a substance, we write its atomic mass and then replace the atomic mass unit u by the word gram or its symbol g.
Atomic mass of oxygen = 16 u
Gram Atomic mass of oxygen = 16g
The gram atomic mass of a substance represents the mass of 1 mole of atoms(6.022×1023)of that substance.
So the number of atoms present in 1 gram atomic mass of any substance is 6.022×1023 atoms.
Leave a Reply