Question 1 How is the size of an atom indicated?
Question 2 Define atomic mass unit?
Question 3 What is the mass of hydrogen atom?
Question 4 Name the element used as a standard for atomic mass scale?
Atomic Mass of an Element
Actual masses of the atoms of the elements are very very small.
For Example : The atom of hydrogen has a mass of 1.6727 x 10-27 kg. It is not convenient to use such small and complicated figures in our calculation,therefore ,it was necessary to define atomic masses in such a way that we get simple figures for them.
Carbon-12 is that atom of carbon which has 6 protons and 6 neutrons in its nucleus so that so its mass number is 12.
carbon-12 atom has been assigned an atomic mass of exactly 12 atomic mass units.This means that a carbon-12 atom has been assigned an atomic mass of exactly 12u.
Atomic Mass unit = the mass of a carbon – 12 atom
One Atomic Unit is defined as exactly one-twelfth the mass of an atom of carbon-12.
Carbon-12 atom is taken to be the standard.The atomic masses of all other elements are determined by comparing the mass of their atom with the mass of a carbon-12 atom.
The atomic mass of an element is relative mass of its atom as compared with the mass of a carbon-12 atom taken as 12 units.
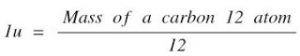
Leave a Reply