Question 1 What happens when crystal of potassium permanganate is dissolved in water ?
Question 2 How will you show that matter us made up of particles ?
Question 3 How will you show that particles of matter are in motion ?
Evidences of particle in matter and their motion
Most of the evidences of particles in matter and their motion comes from the experiments on Diffusion and Brownian movement.
(1) When a crystal of potassium permanganate is placed in a beaker containing water, the water slowly turns purple on its own, even without stirring. Both potassium permanganate crystal and water are made up of tiny particles. The particles of potassium permanganate are purple coloured whereas the particles of water are colourless. When potassium permanganate crystal is put in water, its particles separate from one another. The purple coloured particles of potassium permanganate spread throughout water making the whole water look purple. On dissolving, the particle of potassium permanganate get into the spaces between the particles of the water.

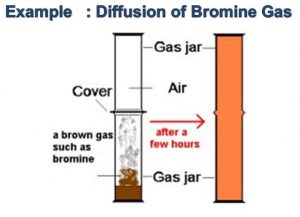
(2) Air is a colourless gas. When a gas jar is empty, it is actually filled with air. Bromine is a red-brown liquid. Bromine vapours is red-brown in colour. A gas jar containing air is placed upside down on a gas jar of bromine vapour. We will see the red-brown vapours of bromine from the lower gas spread up into air in the upper jar and after sometime the gas jar containing air also becomes completed red-brown in colour. Both air and bromine vapour are made of tiny moving particles.
Leave a Reply