Oxides of Nitrogen – The p-Block Elements – Class 12
Contents
Oxides of Nitrogen
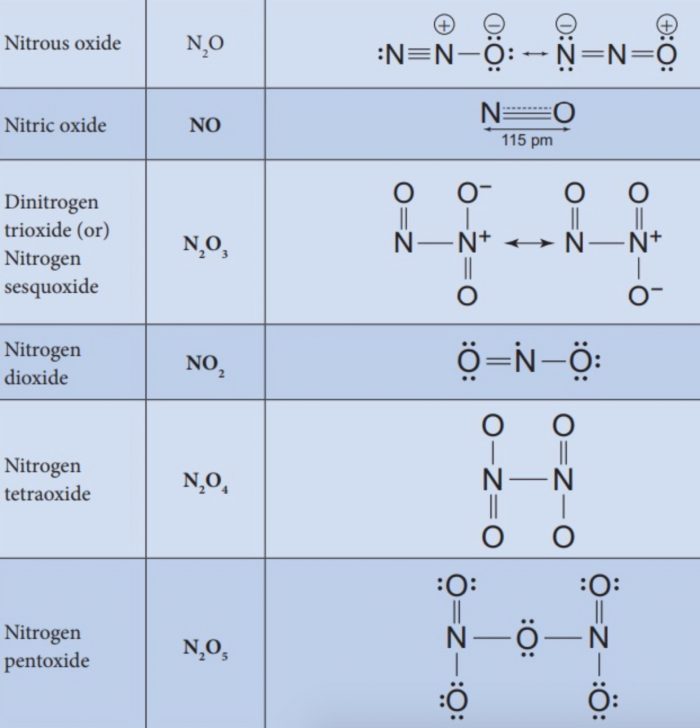
Nitrogen combines with oxygen under different conditions to form a number of binary oxides which differ with respect to the oxidation state of the nitrogen atom. They range from N2O (oxidation state of N +1) through NO (+2), N2O3 (+3), N2O4 (+4) to N2O5(5). The tendency to form pπ- pπ multiple bonds dictates the structures of oxides.
(1) Nitrous Oxide (N2O)
(a) It is prepared by heating ammonium nitrate.
NH4NO3 —–> N2O + 2H20
(b) It is a colourless unreactive gas having faint pleasant smell. It is also known as laughing gas because it causes hysterical laughter when inhaled in minor quantities.
(c) It is a neutral oxide and reacts with sodamide to form sodium azide.
N2O + 2NaNH2 ——–> NaN3 + NH3 + NaOH
Sodamide Sodium azide
(d) In small amounts, it acts as an anaesthetic for minor operations.
(e) It decomposes into nitrogen and oxygen at 873 K.
2N2O ——> 2N2 + O2
Therefore, it supports the combustion acting as a source of oxygen.
(2) Nitric Oxide (NO)
(a) It is prepared by the catalytic oxidation of ammonia at 1100 K in the presence of platinum.
4NH3 + 5O2 ——->4NO + 6H2O
(b) It can also prepared by the reaction of nitric acid on copper as :
3Cu + 8HNO3 ——> 3Cu(NO3)2 + 2NO + 4H2O
(c) It can also be prepared by the reduction of sodium nitrite with ferrous sulphate in the presence of sulphuric acid.
2NaNO2 +2FeSO4 +3H2SO4 ——-> Fe2(SO4)3 + 2NaHSO4 + 2H2O + 2NO
(d) It is a neutral oxide
(e) It is a colourless gas. It has odd number of electrons (11 valence electrons) and therefore, it is paramagnetic in the gaseous state. However, in the liquid and solid states, it forms a loose dimer in such a way that the magnetic effects of two unpaired electrons are cancelled out. The molecule is diamagnetic.
(f) Nitric oxide readily reacts with oxygen to give brown fumes of nitrogen dioxide.
2NO (g) + O2(g) ——–> 2NO2 (g)
(g) Nitric oxide readily forms complexes with transition metals.
For example: Fe2+ combines with NO to form the complex [Fe(H2O)5NO]2+ which is responsible for brown ring test for nitrates.
(h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K)
2NO (g) —–> N2 (g) + O2 (g)
Dinitrogen Trioxide (N2O3)
(1) It is prepared by cooling equimolar quantities of nitric oxide and nitrogen dioxide to below 253 K.
NO(g) + NO2(g) ⇔ N2O3
(2) It can also be prepared by reacting nitric oxide and dinitrogen tetraoxide at 250 K.
2NO + N2O4 ——> 2N2O3
Properties of Dinitrogen Trioxide
(1) It is a blue solid and is acidic in nature. It is anhydride of nitrous acid (HNO2).
N2O3 + H2O —-> 2HNO2
(2) It exists in the pure form only in the solid state at very low temperatures.Above its melting point (273 K) it dissociates to NO and NO2.
N2O3 ——-> NO + NO2
Nitrogen Dioxide (NO2)
It is prepared by heating dried lead nitrate in a steel reaction vessel.
2Pb(NO3)2 ——->2PbO+ 4NO2 + O2
It is also an odd electron molecule and in the gas phase, it exists in equilibrium with N2O4 as :
N2O4 ⇔ 2 NO2
Above 415 K it contains mainly NO2 and at 250 K, it consists of mainly N2O4
2 NO2 ⇔ N2O4
Dinitrogen Pentoxide (N2O5)
(1) It is prepared by dehydrating the concentrated nitric acid with phosphorus pentoxide.
4HNO3 + P4O10 —–> 2N2O5 + HPO3
(2) N2O5 exists as colourless solid below 273K. As the temperature rises, the colour changes to yellow due to the partial decomposition of colourless N2O5 to brown NO2.
2 N2O5 ——–> 4NO2 + O2
(3) At 303K, the crystals melt giving a yellow liquid which decomposes at 313K to give NO2.
(4) N2O5 acts as a strong oxidising agent and oxidises iodine to I2O5
NO and NO2 are used in the manufacture of nitric acid and nitrate fertilizers Liquid N2O4 is also used as an oxidiser for the rocket fuels in missiles and space vehicles.
(5) NO causes a pollution problem in atmosphere due to its poisonous nature. Its vapours are emitted in the atmosphere during the burning of oil and coal.
Leave a Reply