Contents
Preparation of Lyophilic Colloids
The lyophilic colloids have strong affinity between particles of dispersed phase and dispersion medium. Therefore, these colloidal solutions are readily formed by simply mixing the dispersed phase and dispersion medium under ordinary conditions.
For example: The substances like gelatin, gum, starch, egg albumin etc. pass readily into water to give colloidal solution.
They are reversible in nature because these can be precipitated and directly converted into colloidal state.
Lyophobic sols can be prepared by mainly two types of methods:
1) Condensation methods
2) Dispersion methods.
Condensation Methods
In these methods, smaller particles of dispersed phase are condensed suitably to be of colloidal size. This is done by the following methods :
a) By chemical Reactions
The chemical reactions given ahead may be used to prepare lyophobic colloidal solutions.
(1) Oxidation: A colloidal sol of sulphur is obtained by bubbling H2S gas through the solution of bromine water, sulphur dioxide, etc.
H2S + Br2 —-> 2HBr + S
2 H2S + SO2 ——> 2H2O + 3S
(2) Reduction: The colloidal solutions of metals are obtained by reduction of their compounds.
For example: a solution of AuCl3 is reduced with SnCl2.
2 AuCl3 + 3 SnCl2 ———> 3 SnCl4 + 2 Au
The reaction can also be carried out with formaldehyde.
2 AuCl3 + 3HCHO + 3 H2O ——> 2 Au + 3 HCOOH + 6 HCl
The gold sol, thus prepared, has a purple colour and is called purple of cassius.
(iii) Hydrolysis: A colloidal solution of ferric hydroxide is prepared when a concentrated solution of ferric chloride is added drop wise to hot water.
FeCl3 +3H2O ——> Fe(OH)3 +3HCl
(iv) Double decomposition: As2S3 sol is obtained by passing H2S through dilute solution of arsenious oxide in water.
As2O3 + 3H2S —–> As2S3 +3H20
(b) By Excessive Cooling
A colloidal solution of ice in an organic solvent like ether or chloroform can be prepared by freezing a solution of water in the solvent. The molecules of water which can no longer be held in solution, separately combine to form particles of colloidal size.
(c) By Exchange of Solvent
Colloidal solution of certain substances such as sulphur, phosphorus which are soluble in alcohol but insoluble in water can be prepared by pouring their alcoholic solution in excess of water.
(d) By change of Physical State
Sols of substances like mercury and sulphur are prepared by passing their vapours through a cold water containing a suitable stabilizer such as ammonium salt or citrate.
Dispersion Methods
In these methods, larger particles of a substance (suspension) are broken into smaller particles.
(a) Mechanical dispersion
In this methods, the substance is first ground to coarse particles. It is then mixed with the dispersion medium to get a suspension. The suspension is then grinded in a colloidal mill . It consists of two metallic discs nearly touching each other and rotating in opposite directions at a very high speed.
The space between the discs of the mill is so adjusted that coarse suspension is subjected to great shearing force giving rise to particles of colloidal size. Colloidal solutions of black ink, paints, varnishes, dyes, etc. are obtained by this method.
(b) By Electrical Dispersion or Bredig’s arc Method
This method is used to prepare sols of metals such as platinum, silver, copper or gold.
The metal whose sol is to be prepared is made as two electrodes immersed in dispersion medium such as water. The dispersion medium is kept cooled by surrounding it with a freezing mixture. An electric arc is struck between the electrodes. The tremendous heat generated by the arc vaporises the metals which are condensed immediately in the liquid to give colloidal solution. The colloidal solution prepared is stabilised by adding a small amount of KOH to it.
(c) By Peptization
The process of converting a freshly prepared precipitate into colloidal form by the addition of a suitable electrolyte is called peptization.
The electrolytes used for the purpose are called peptising agents..
Cause of Peptization
When an electrolyte is added to a freshly prepared precipitate, the suitable ions from the added electrolyte are adsorbed by the particles of the precipitate. The charged particles repel one another and form colloidal solution.
For example: On treating a precipitate of iron (III) oxide with a small amount of FeCl3 solution, gives a reddish brown coloured colloidal solution.
Fe(OH)3 + Fe3+ ——–> Fe(OH)3Fe3+
A precipitate of silver chloride can be peptised by shaking with a dilute solution of silver nitrate to give a colloidal solution of silver chloride.
AgCl + Ag+ ——-> AgCl.Ag+
Purification of Colloidal Solution
Impurities especially electrolytes which can destabilize the sols must be eliminated to make the colloidal solutions stable.
The following methods are commonly used for the purification of colloidal solutions.
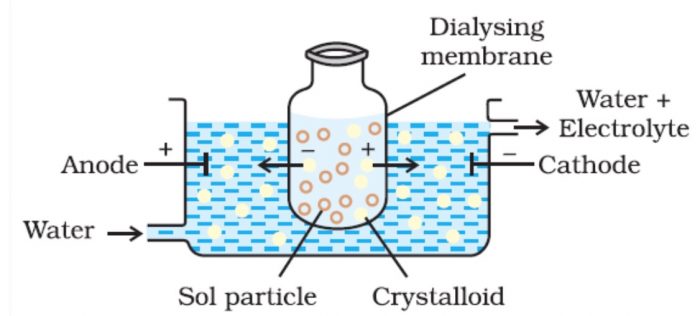
1) Dialysis
The process of separating the particles of colloids from those of crystalloids by means of diffusion through a suitable membrane is called dialysis.
Principle : Colloidal particles cannot pass through a parchment of cellophane membrane while the ions of the electrolyte can pass through it.
a) The colloidal solution is taken in a bag made of cellophane or parchment.
b) The bag is suspended in fresh water. The impurities slowly diffuse out of the bag leaving behind pure colloidal solution .
c) The distilled water is changed frequently to avoid accumulation of the crystalloids otherwise they may start diffusing back into the bag.
d) Dialysis can be used for removing HCl from the ferric hydroxide sol.
e) To increase the process of purification , the dialysis is carried out by applying electric field.This process is called electrodialysis.
2) Ultra-filtration
It is the process of removing the impurities from the colloidal solution by passing it through graded filter papers called ultra-filter papers.
a) These filter papers are permeable to all substances except colloidal particles. Colloidal particles can pass through ordinary filter paper because the size of the pores is too large.
b) However, the size of the pores of filter paper can be reduced by impregnating them with colloidion or gelatin solution to stop the flow of colloidal particles.
c) The colloidion solution generally used is 4% solution of nitrocellulose in a mixture of alcohol and ether.
d) An ultra-filter paper may be prepared by soaking the filter paper in a colloidion solution, hardening by dipping in formaldehyde solution and then finally drying it.
e) With these ultra-filter papers impurities of different sizes can be effectively removed. In this method, sol is poured over the ultrafilters which allows solution of impurities to pass through but retains the colloidal particles.
f) The colloidal particles left on the ultra-filter paper are then stirred with fresh dispersion medium (solvent) to get a pure colloidal solution. This is a slow process and to speed up the process, pressure or suction is applied.
3) Ultra-Centrifugation
In this method, the colloidal sol is taken in a tube which is placed in an ultra centrifuge.
On rotation of the tube at high speeds, the colloidal particles settle down at the bottom of the tube and the impurities remain down in the solution called centrifugate.
The settled colloidal particles are mixed with an appropriate dispersing medium to regenerate the sol.
Leave a Reply