Question 1 What is sublimation.
Question 2 Define sublimate.
Question 3 Define sublime.
Question 4 Give few examples of substances that undergoes sublimation ?
Question 5 Why naphthalene balls kept in stored clothes in our homes disappear over a period of time ?
Question 6 How does applying pressure help in the liquefaction of a gas ?
Question 7 What is the effect of pressure on change in state of matter?
Question 8 What is solid carbon dioxide?
Question 9 Why solid carbon dioxide is called dry ice ?
Sublimation
- sublimation
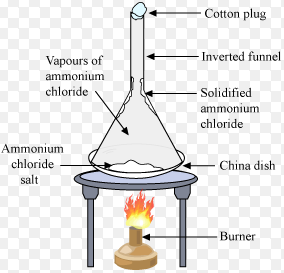
The Changing of a solid directly into vapours on heating and of vapours into solid on cooling is called as sublimation.
The solid substance which undergoes sublimation is called sublime.
The solid obtained by cooling the vapours of the solid called a sublimate.
For Example :camphor, Iodine, Ammonium Chloride, Naphthalene etc.
Effect of Change of Pressure
The physical state of matter can be changed by increasing or decreasing the pressure. When a high pressure is applied to a gas, it gets compressed and when we lower its temperature, it gets liquified. Gases can be liquified by compression and cooling.
For Example: Solid carbon dioxide is stored under high pressure. This is because on decreasing the pressure on solid carbon dioxide, it gets converted directly into carbon dioxide gas.
I’m more than happy to uncover this site. I want to to thank you for ones time for this particularly wonderful read!! I definitely savored every part of it and I have you saved as a favorite to check out new stuff in your website.
Thanks Bernardina
I found it good package fr learning and teaching as well. V well illustrated diagrams.
Thank you so much Jasmeet for your wonderful comments.
Excellent post. Keep posting such kind of info on your page.
Im really impressed by your site.
Hey there, You’ve done an excellent job. I will definitely digg it
and in my view suggest to my friends. I’m sure they will be benefited
from this site.
Wow, that’s things i was seeking for, what a material! existing at
this weblog, thanks admin with this website.
Great site you may have here.. It’s difficult to get high-quality writing like
yours today. I truly appreciate individuals such as you! Take
care!!
WOW just what I was searching for. Came here by searching for articles
Good article! We are linking to the particularly great article on our website.
Continue the best writing.
Wow Thanks 4 sharing this amazing information …
Thank you so much for posting this wonderful answer. Admin. It helped me a lot in my studies. I need you to keep on posting such informative answers. Thanks again
Thanks for wonderful answer
Thanks for sharing this wonderful piece of information.