Question 1 What is the chemical name and formula of washing soda?
Question 2 What is soda ash?
Question 3 Name a metal compound which has detergent properties?
Question 4 Name a sodium compound used for making borax and glass?
Question 5 Explain the preparation of washing soda by solvay’s process?
Question 6 Give uses of washing soda?
Question 7 Give few properties of washing soda?
Question 2 What is soda ash?
Question 3 Name a metal compound which has detergent properties?
Question 4 Name a sodium compound used for making borax and glass?
Question 5 Explain the preparation of washing soda by solvay’s process?
Question 6 Give uses of washing soda?
Question 7 Give few properties of washing soda?
Contents
Washing Soda
Washing soda is sodium carbonate containing 10 molecules of water of crystallisation.It is called as sodium carbonate decahydrate.
The formula of washing soda is
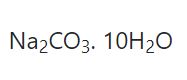
Sodium carbonate which does not contain any water of crystallisation is called anhydrous sodium carbonate or soda ash.The formula is Na2CO3
Preparation of Washing Soda
It is prepared by Solvay’s process.
(1) A cold and concentrated solution of sodium chloride is reacted with ammonia and carbon dioxide to obtain sodium hydrogen carbonate.
(2) Sodium hydrogen carbonate is separated by filtration,dried and heated.Oh heating sodium hydrogen carbonate decomposes to form sodium carbonate.
(3) Anhydrous sodium carbonate is dissolved in water and recrystallised to get washing soda crystals containing 10 molecules of water of crystallisation.
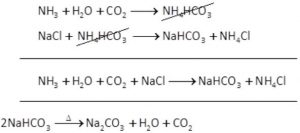
Properties of Washing Soda
(1) It is a white transparent crystalline solid.
(2) It is soluble in water.
(3) The solution of washing soda in water is alkaline which turns red litmus to blue.
(4) It has cleansing properties.
Uses of Washing Soda
(1) It is used to remove permanent hardness of water.
(2) It is used in the manufacture of glass,soap,paper.
(3) It is used for washing clothes.
(4) It is used in the manufacture of borax.
Good
Nice answer
Nice one
this is good one
Nice good answer
THANKS!! FOR THE ANSWER
Good presentation
Thank you for answer
Helpful
Thanks mam
Brillant answers
Nice and best answer.
Excellent
Great explanation….superb