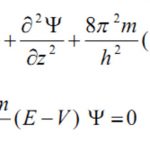Quantum mechanics, as developed by Erwin Schrodinger in 1926, is based on the wave motion associated with the particles. For the wave motion of the electron in the three dimensional space around the nucleus, he put forward an equation known as Schrondinger wave equation. where ψ is the amplitude of the wave where the coordinates of the electrons are ( x,y,z) ,E is the … [Read more...] about Quantum Mechanical Model of an Atom