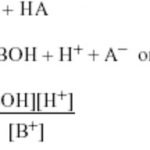Salt hydrolysis is defined as the process in which a salt reacts with water to give back the acid and the base. Salt +water ----------> Acid + Base BA + H2O ---------> HA + BOH All salts are strong electrolytes and thus ionize completely in the aqueous solution. (1) If the acid produced is strong and the base produced is weak. B+ + A‾ + H2O -------> H+ + … [Read more...] about Salt Hydrolysis