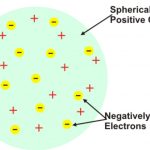
Thomson's model of atom J.J. Thomson in 1904, proposed that an atom was a sphere of positive electricity in which were embedded number of electrons. The stability of the atom was explained as a result of the balance between the repulsive forces between the electrons and their attraction towards the centre of the positive Sphere.This model is compared with a watermelon … [Read more...] about Rutherford’s Model of an Atom
rutherford model of an atom
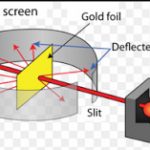
Rutherford Model of an Atom
Question 1 Name the particle used by Rutherford in his experiment to determine the structure of an atom? Question 2 Explain the Rutherford's scattering experiment? Question 3 What observation were made by Rutherford's in his experiment? Question 4 Give the postulates of Rutherford's model of an atom? Question 5 State one drawback of Rutherford's model of an … [Read more...] about Rutherford Model of an Atom