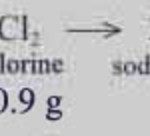
Law of Conservation of Mass This Law was studied by French chemist Antoine lavoisier in 1789 This law states that In all physical and chemical changes, the total mass of the reactants is equal to that of the products. Or Mass can neither be created nor destroyed. This law is also called as the law of indestructibility of matter. The mass and energy are … [Read more...] about Laws of Chemical Combination