Law of Conservation of Mass This Law was studied by French chemist Antoine lavoisier in 1789 This law states that In all physical and chemical changes, the total mass of the reactants is equal to that of the products. Or Mass can neither be created nor destroyed. This law is also called as the law of indestructibility of matter. The mass and energy are … [Read more...] about Laws of Chemical Combination
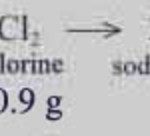
Law of Constant Proportion
Atoms and Molecules
Question 1 Which Indian philosopher suggested that all matter is composed of ver small particles. What name was given by him to these particles? Question 2 What is law of conservation of mass? Give example? Question 3 What is meant by law of constant proportion? Give example? Question 4 Name the scientist who gave law of conservation of mass? Question 5 Name the … [Read more...] about Atoms and Molecules