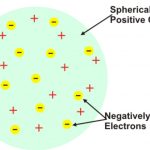
Thomson's model of atom J.J. Thomson in 1904, proposed that an atom was a sphere of positive electricity in which were embedded number of electrons. The stability of the atom was explained as a result of the balance between the repulsive forces between the electrons and their attraction towards the centre of the positive Sphere.This model is compared with a watermelon … [Read more...] about Rutherford’s Model of an Atom
isotopes
Dalton’s Atomic Theory
Dalton's Atomic Theory John Dalton in 1808 put forward theory known as Dalton's atomic theory The main points of this theory are: (1) Matter is made up of extremely small indivisible particles called atoms. (2) Atoms of the same element are identical in all respect i.e. shape, size and mass. (3) Atoms of different elements have different masses, sizes and also … [Read more...] about Dalton’s Atomic Theory
Isotopes
Question 1 What are isotopes? Question 2 Give examples of isotopes? Question 3 Give few application of isotopes? Question 4 why isotopes have same chemical properties? Question 5 Why isotopes have different physical properties? Isotopes Isotopes are atoms of same element having same atomic number but different mass numbers. For Ex: 1)Isotopes of … [Read more...] about Isotopes
Dalton’s Atomic Theory
Question 1 Name the scientist who gave atomic theory of matter? Question 2 State the various postulates of Dalton's Atomic theory of matter? Question 3 Give drawbacks of Dalton's Atomic theory of matter? Dalton's Atomic Theory Dalton put forward his atomic theory of matter in 1808. Postulates (1) All the matter is made up of very small particles called atoms. (2) … [Read more...] about Dalton’s Atomic Theory