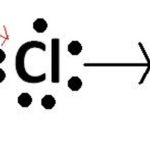1) All the alkali metals are highly reactive elements since have a strong tendency to lose the single valence s-electron to form unipositive ions having inert gas configuration. 2) This reactivity arises due to their low ionisation enthalpies and high negative values of their standard electrode potential. 3) Due to their strong tendency to lose the single valence electron … [Read more...] about Chemical properties of s-block elements
ionisation enthalpy
Ionic Bond
When a bond is formed by complete transference of electrons from one atom to another so as to complete their outermost orbit by acquiring 8 electrons or 2 electrons in case of hydrogen, lithium etc and hence acquire the stable nearest noble gas configuration, the bond form is called ionic bond or electrovalent bond. On losing an electron, an atom becomes positively charged … [Read more...] about Ionic Bond