Oxidation State The elements of group 13 have to two electrons in the s- orbital and one electron in the p-orbital of the valence shell. These elements are expected to show a uniform oxidation state of +3. Boron and aluminium which show an oxidation state of +3 only but gallium, indium and thallium due to inert pair effect show oxidation state of both +1 and +3 . As we … [Read more...] about Chemical Properties of Boron Family
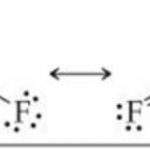
inert pair effect
General Characteristics of p-Block elements
Elements in which the last electron enters any one of the three p orbitals of their respective outermost shells are called p block elements. A p-subshell has three degenerate p-orbitals, each of which can accommodate 2 electrons, therefore, in all, there are six groups of p-block elements i.e. group 13,14,15, 16, 17 and 18 each containing 6 elements. The atoms of elements … [Read more...] about General Characteristics of p-Block elements