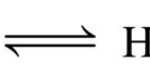
For Acidic Buffer mixture( Henderson- Hasselbalch equation) For Basic Buffer mixture pOH = pKb + log [salt] / [Base] pH + pOH =14 pOH = 14 - pH pKa + pKb = 14 pKb=14 - pKa 14 - pH = 14 - pKa + log [salt) / [base] pH = pKa - log [salt] / [base] pH = pKa + log [base] / [salt] where Ka is the ionization constant of the conjugate acid of the … [Read more...] about Calculation of pH of a Buffer Mixture