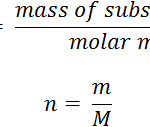Mole Concept Some Basic Concepts of Chemistry Class 11 Avogadro's number or Avogadro's constant (NA) One gram atom of any element contains the same number of atoms and one gram molecule of any substance contains the same number of molecules. The value was found to be 6.022137 × 1023 The value generally used is 6.022 × 1023 . This is called Avogadro's number … [Read more...] about Mole Concept
avogadro's number
Mole Concept
Question 1 Define a mole? Question 2 What is the value of Avogadro's constant? Question 3 Define mole of an atom? Question 4 Define mole of a molecule? Question 5 Write the formula to calculate the number of moles? Mole Concept In a chemical reaction equation,it is more convenient to use quantity of substance in number of its molecules or atoms rather … [Read more...] about Mole Concept