Class 9 Science Chapter 2 |
Is Matter Around Us Pure NCERT Solutions
Page 15
1.What is meant by a substance?
Answer:
A pure substance is the one that consist of a single type of particle i.e. all constituent particles of the substance have the same chemical nature.
2. List the points of differences between homogeneous and heterogeneous mixtures.
Answer:
| HOMOGENEOUS MIXTURE |
HETEROGENEOUS MIXTURE |
| 1)Those mixtures in which the substances are completely mixed together and are indistinguishable from one another. | Those mixtures in which the substances remain separate and one substance is spread throughout the other. |
| 2)They have uniform composition throughout its mass. | They do not have uniform composition. |
| 3) It has no visible boundaries of separation between various constituents. | It has visible boundaries. |
Page 18
1. Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:
| HOMOGENEOUS MIXTURE |
HETEROGENEOUS MIXTURE |
| 1)Those mixtures in which the substances are completely mixed together and are indistinguishable from one another. | Those mixtures in which the substances remain separate and one substance is spread throughout the other. |
| 2)They have uniform composition throughout its mass. | They do not have uniform composition. |
| 3) It has no visible boundaries of separation between various constituents. | It has visible boundaries. |
| 4) For Ex: Sugar solution, salt solution, alcohol and water, Soft drinks etc. | For Ex: Sugar and sand, Salt and Sand, Milk, Soil, Blood, Starch, Muddy water etc. |
2. How are sol, solution and suspension different from each other?
Answer:
Sol is a heterogenous mixture in which the solute particles are so small that they cannot be seen with naked eyes. They seem to be spread uniformly throughout the mixture. They show tyndall effect.
For ex: Milk of magnesia, mud
Solution is a homogenous mixture in which solute particles dissolve and spread uniformly throughout the mixture. Solutions do not show Tyndall effect.
For ex: Salt in water, sugar in water, iodine in water
A suspension is heterogenous mixture in which small particles of a solid are spread throughout a liquid without dissolving in it.
For ex: Chalk and water, muddy water, sand and water, flour and water.
3. To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Mass of solute (sodium chloride) = 36 g (given)
Mass of solvent (water) = 100g (given)
Then, mass of solution = Mass of solute + Mass of solvent
=(36+100) g
= 136 g
Therefore, concentration (mass by percentage) of the solution
= (Mass of Solute/Mass of solvent) x 100%
= 36/136 x 100%
= 26.4%
Page 24
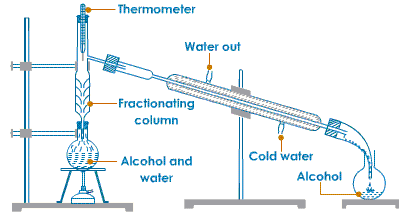
1. How will you separate a mixture containing kerosene and petrol (difference in their boiling points
is more than 25ºC), which are miscible with each other?
Answer:
A mixture of two liquids having a difference in boiling point more than 25ºC can be separated by the method of distillation.
In this method, the mixture of kerosene and petrol is taken in a distillation flask with a thermometer fitted in it. We need a beaker, a water condenser and a bunsen burner. Then the apparatus is set as shown in the diagram. The mixture is then slowly heated. Kerosene will vaporise and condense in the water condenser. The condensed kerosene is collected from the condenser outlet, whereas petrol is left behind in the distillation flask.
2. Name the technique to separate
(i) butter from curd,
(ii) salt from sea-water,
(iii) camphor from salt.
Answer:
a) Centrifugation
b) Evaporation
c) Sublimation
3. What type of mixtures are separated by the technique of crystallisation?
Answer:
The process of cooling a hot,concentrated solution of a substance to obtain crystals is called crystallisation.
It is used for obtaining a pure solid substance from impure sample.
For Ex: Impure copper sulphate can be purified by the method of crystallisation.
Page 24
1. Classify the following as chemical or physical changes:
• cutting of trees,
• melting of butter in a pan,
• rusting of almirah,
• boiling of water to form steam,
• passing of electric current, through water and the water breaking down into hydrogen and oxygen gases,
• dissolving common salt in water,
• making a fruit salad with raw fruits, and
• burning of paper and wood.
Answer:
Cutting of trees : Physical change
Melting of butter in a pan: Physical change
rusting of almirah: Chemical change
Boiling of water to form steam: Physical change
Passing of electric current through water and the water breaking down into hydrogen and oxygen gases: Chemical change
Dissolving common salt in water: Physical change
Making a fruit salad with raw fruits: Physical change
Burning of paper and wood : Chemical change
2. Try segregating the things around you as pure substances or mixtures.
Answer:
Pure substances : salt, water, sugar
Mixtures: soil, wood, air, book, chair , cold drink, milk, butter, food
Page 28
1. Which separation techniques will you apply for the
separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium
chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
a) Evaporation
b) Sublimation
c) Centrifugation or Filtration
d) Chromatography
e) Centrifugation
f) Separating funnel
g) Simple filtration
h) Magnetic separation
i) Winnowing
j) Centrifugation
2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:
1)Water , the solvent is taken in a kettle. This water is allowed to boil.
2) During heating, sugar and tea leaves are added as solute.
3) Milk , tea leaves and sugar together form solution. Sugar dissolves in milk.
4) Then the solution is poured on a strainer.
5) The remaining tea leaves being insoluble remains as residue.
6) Colour of the tea leaves goes into solution as filtrate.
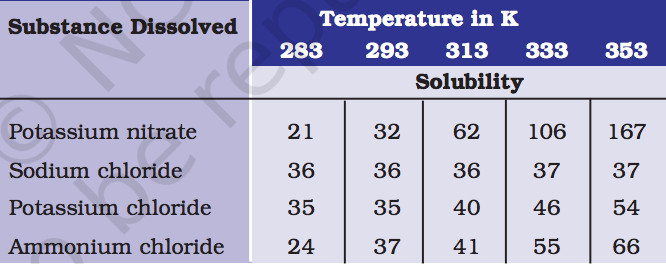
3. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
Answer:
At 313 K
Mass of potassium nitrate needed to produce its saturated solution in 100g of water is 62 g.
Mass of potassium nitrate needed to produce its saturated solution in 50g of water is 31 g.
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools?Explain.
Answer:
The amount of potassium chloride that should be dissolved in water to make a saturated solution increases with temperature. As the solution cools down some amount of dissolved potassium chloride will reappear as undissolved solid.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
Answer:
Solubility of salt at 293 K are:
a) Potassium nitrate- 32 g
b) Sodium chloride- 36 g
c) Potassium chloride-35 g
d) Ammonium chloride -37 g
Ammonium chloride has the highest solubility.
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:
The solubility of salt increases with temperature.
4. Explain the following giving examples.
(a) saturated solution
Answer:
A solution in which no more quantity of solute can be dissolved at given temperature is called saturated solution.
Suppose 100 g of a solute is the maximum amount that can be dissolved in 200 g of water at 298 K. Then 300 g of solution so obtained is the saturated solution at 298 K.
(b) pure substance
A pure substance is one which is made up of only one kind of particle(atoms or molecules)
For ex: Sulphur element is made up of only one kind of sulphur atom.
Water is made up of only one kind of water molecule.
(c) colloid
Answer:
A solution in which the size of solute particles is intermediate between those in true solution and suspension.
For ex: milk, blood, starch solution, soap solution.
(d) suspension
Answer:
A suspension is a heterogenous mixture in which small particles of a solid are spread throughout a liquid without dissolving in it.
For ex: muddy water, chalk water, sand water etc.
5. Classify each of the following as a homogeneous or heterogeneous mixture.
soda water, wood, air, soil, vinegar, filtered tea.
Answer:
Homogeneous mixture: Soda water, air, vinegar, filtered tea
Heterogenous mixture: wood, soil
6. How would you confirm that a colourless liquid given to you is pure water?
Answer:
Every liquid has a characteristic boiling point at 1 atmospheric pressure. Pure water has a boiling point of 373 K at 1 atmospheric pressure.If the colourless liquid boils exactly at 373 K at 1 atmospheric pressure then it is a pure water. If there is difference in boiling point then water is contaminated.
7. Which of the following materials fall in the category of a “pure
substance”?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air.
Answer: a, c, d, e and f are pure substances.
8. Identify the solutions among the following mixtures.
(a) Soil
(b) Sea water
(c) Air
(d) Coal
(e) Soda water.
Answer: Solution among mixtures are:
a) Sea water
b) Air
c) Soda water
9. Which of the following will show “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution.
Answer: b and d are colloids which show Tyndall effect
10. Classify the following into elements, compounds and mixtures.
(a) Sodium
(b) Soil
(c) Sugar solution
(d) Silver
(e) Calcium carbonate
(f) Tin
(g) Silicon
(h) Coal
(i) Air
(j) Soap
(k) Methane
(l) Carbon dioxide
(m) Blood
Answer: Elements: Sodium, silver, tin, silicon
Compounds: Calcium carbonate, methane, carbon dioxide
Mixtures: Soil, coal, air, soap, blood, sugar solution
11. Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle.
Answer: The following changes are chemical changes:
a) Growth of plant
b) Rusting of iron
c) Cooking of food
d) Digestion of food
e) Burning of candle
Leave a Reply