Question 1 What is a physical change?
Question 2 Give few examples of physical changes from everyday life?
Question 3 Explain why melting of ice to form water is said to be physical change?
Question 4 What are the characteristics of physical changes?
Question 5 Explain why boiling of water is said to be physical change?
All substances have certain properties such as state (solid, liquid or gas), size, shape, colour, smell, temperature, composition and structure, etc. When one or more properties of a substance become different, we say that a change has taken place in it.
Melting of ice is a common change around us. Ice is a solid whereas water is a liquid. So, the melting of ice involves a change in state from solid state to liquid state.
Some of the changes observed by us in our everyday life are:
(1) Formation of curd from milk
(2) Cooking of food
(3) Burning of fuels
(4) Drying of clothes
(5) Ripening of fruits, and
(6) Rusting of iron
The change in a substance does not occur on its own. There is always a cause which brings about a change in a substance. Ice does not melt on its own to form water. Ice must be given some heat to melt and change into water. Thus, heat is the cause of the change of state of ice from solid to liquid.
Contents
Types of Changes
Changes can be of two types:
1) Physical changes, and
2) Chemical changes
Physical changes
Those changes in which no new substances are formed, are called physical changes. The changes in state, size, shape and colour of a substance are physical changes. The properties such as state, size, shape and colour of a substance are called its physical properties.
Those changes in which a substance undergoes a change in its physical properties are called physical changes.
The important characteristics of a physical change are as follows:
(1) No new substance is formed in a physical change.
(2) A physical change is a temporary change. A physical change can be easily reversed.
(3) Very little energy is either absorbed or evolved in a physical change.
(4) A temporary change in colour may take place in a physical change.
Examples of physical changes :
Melting of ice (to form water)
Freezing of water (to form ice)
Boiling of water (to form steam
Condensation of steam (to form water Evaporation of water to form water vapour)
Condensation of water vapour (to form liquid water)
Cutting of cloth
Conversion of chalk stick into chalk dust
Breaking of a glass tumbler
Breaking of a wooden stick
Cutting of a log of wood (into pieces of wood)
Drying of wet clothes
Dissolving salt in water (to make salt solution)
Dissolving sugar in water (to make sugar solution)
Making soda water by dissolving carbon dioxide
Glowing of an electric bulb (or tube-light)
Stretching of a rubber band
Grinding of a substance
Hammering of metals to form thin sheets
Melting of wax
Formation of clouds
Stretching metals to form wires
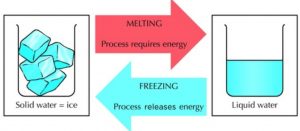
Melting of Ice and Freezing of Water
Take some ice in a beaker and keep it aside for some time. We will see that ice melts to form water. The ice kept in beaker receives heat from the surrounding air to melt and form water.
Though ice and water look different, they are both made of water molecules. No new substance is formed during the melting of ice, only a change of state (from solid to liquid) takes place during the melting of ice.
So, the melting of ice (to form water) is a physical change.
The change which occurs during the melting of ice to form water can be reversed easily by freezing the water to form ice again.
Let us keep the beaker containing water in the freezer compartment of a refrigerator. After a few hours, the water kept in the freezer of a refrigerator gets cooled too much, freezes (or solidifies) to form ice. The liquid water changes into solid water called ice. Only a change in state (from liquid to solid) takes place during the freezing of water to form ice, but no new substance is formed. So, the freezing of water (to form ice) is a physical change.
Boiling of Water and Condensation of Steam
Take some water in a hard glass beaker and heat it by using a burner till it starts boiling. When the water starts boiling, we can see the steam rising from the surface of hot water. Water is a liquid whereas steam is a gas. So, during the boiling of water, only a change in state (from liquid to gas) has taken place. Though water and steam look different, they are both made of water molecules. No new substance is formed during the boiling of water.
So, the boiling of water (to form steam) is a physical change.
Hold an inverted frying pan by its handle over the rising steam at some distance above the beaker of boiling water. Now, if we look at the inner surface of the frying pan, we will see drops of water sticking to it. Actually when hot, rising steam comes in contact with the inverted frying pan, then some of the steam gets cooled and condenses to form drops of liquid water. During the condensation of steam, there is only a change in state from gaseous state to liquid state but no new substance is formed.
So, the condensation of steam (to form water) is a physical change.
Chalk Stick and Chalk Dust
The conversion of chalk stick into chalk dust is a physical change because both the chalk stick and the chalk dust are just the same substance, only their size is different. No new substance is formed during the conversion of chalk stick into chalk dust.
We take the chalk dust (or chalk powder) and add a little water to it to make a thick paste of chalk dust. This thick paste of chalk dust can be moulded into a chalk stick and then dried. In this way, we can get back the original chalk stick from the chalk dust.
Making of a Solution
We take some water in a porcelain dish and dissolve some common salt in it. The salt disappears in water and forms a salt solution. Heat this porcelain dish containing salt solution on a burner till all the water evaporates. A white powder is left behind in the porcelain dish. If we taste this white powder, we will find that it is common salt.
No new chemical substance has been formed by dissolving common salt in water to make salt solution. So, the dissolving of salt in water (to make salt solution) is a physical change.
Breaking of a Glass Tumbler
When a glass tumbler breaks, it forms many pieces. Each broken piece of glass tumbler is still glass. So during the breaking of a glass tumbler, only the size and shape of glass has changed but no new substance has been formed. So, the breaking of a glass tumbler is a physical change.
Very nice explanation…
so good and simple
Very simple,I like that ☺️.
Very nice and simple
I have read it all and the explanation is very good but I can’t copy paste it actually my chemistry project that will be in ppt that’s why I need to copy and paste it pls remove the setting that you have done and if you have made any setting then just reply we have not done anything like this I really need this or I will have to type 🙁