Question 1 State Modern Periodic Law?
Question 2 How many groups and periods are there in Modern periodic table?
Question 3 What was the basis of Modern periodic table?
Question 4 Define the term group and period in Modern periodic table?
Question 5 Define periodicity?
Question 6 Explain Long from of Periodic table?
Question 7 How were the position of isotopes of an element decided in the Modern periodic table?
Question 8 What are the merits of Modern periodic table?
Contents
Modern Periodic Law
In the year 1913, Henry Moseley, a young physicist from England studied the frequencies of the X-rays which were emitted when certain metals were bombarded with high speed electrons. He found that in all the cases, the square root of the frequency was directly proportional to the atomic number of the atom of the metal. He further stated that there was no co-relation between the frequency and the atomic mass.
When Moseley plotted a graph between square root of frequency and atomic numbers of different metals, a straight line was obtained. But it was not the case when a graph was plotted between square root of frequency and atomic masses of the metals. These studies led Moseley to believe that atomic number and not the atomic mass is, the fundamental property of an element.
According to him, atomic numbers must form the basis of the classification of the elements in the periodic table.
Moseley gave the Modern Periodic Law which states that: Physical and chemical properties of the elements are the periodic function of their atomic numbers.
Atomic mass of an element is due to the mass of protons and neutrons present in the nucleus of its atom.
As the number of electrons in an atom are given by the atomic number and not by the mass number therefore, atomic number should form the basis of the classification of the elements in the periodic table and not atomic mass as predicted by Mendeleev.
Cause of Periodicity: Periodicity may be defined as the repetition of the similar properties of the elements placed in a group and separated by certain definite gaps of atomic numbers.
Electronic configuration of Alkali metals
| Element | Atomic Number | Electronic Configuration |
| Lithium | 3 | 2,1 |
| Sodium | 11 | 2,8,1 |
| Potassium | 19 | 2,8,8,1 |
| Rubidium | 37 | 2,8,18,8,1 |
| Cesium | 55 | 2,8,18,18,8,1 |
| Francium | 87 | 2,8,18,18,32,8,1 |
It is clear that all of them have one electron each in the valence shell of their atoms.
Electronic configuration of Halogens
| Element | Atomic Number | Electronic Configuration |
| Fluorine | 9 | 2,7 |
| Chlorine | 17 | 2,8,7 |
| Bromine | 35 | 2,8,18,7 |
| Iodine | 53 | 2,8,18,18,7 |
| Astatine | 85 | 2,8,18,32,18,7 |
All the elements included in the group have seven electrons in the valence shell of their atoms.
The resemblance in properties of the elements (particularly the chemical properties) is because of the repetition of the same valence shell electronic configuration.
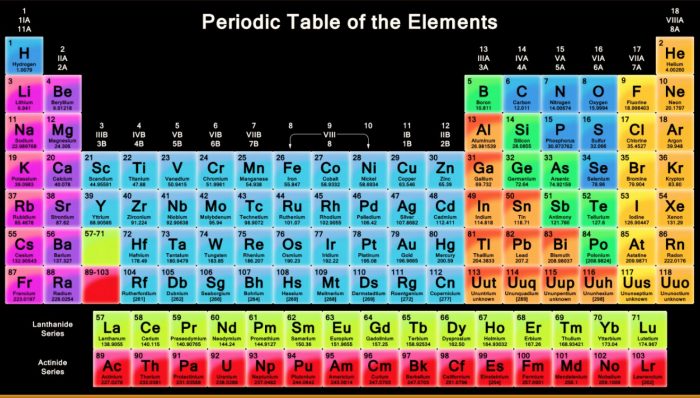
Modern Periodic Table
Modern Periodic Table is based on the Modern Periodic Law. The elements are arranged in order of increasing atomic numbers.
(1) Groups: There are eighteen (18) groups in the Modern Periodic Table. These are numbered from 1 to 18.
(1) The elements present in a group are separated by definite gaps of atomic numbers (8, 8, 18, 18, 32).
(2) The elements present in a group have the same number of electrons in the valence shell of their atoms.
(3) The elements present in a group have identical chemical properties.
(4) The physical properties of the elements in group such as melting point, boiling point, density vary gradually.
(5) Atomic radii of the elements present in a group increase downwards.
(2) Periods: Modern Periodic Table has seven (7) periods also called horizontal rows.
(1) First period has two elements and is called short period.
(2) Second & third periods have eight elements and are also called short periods.
(3)Fourth and fifth periods have eighteen elements and are called long periods.
(4)Sixth and seventh periods have thirty two elements and are also called long periods.
The important characteristics of the elements in a period
(1) In all the elements present in a period, the electrons are filled in the valence shell.
(2) As the number of electrons in the valence shell change, the chemical properties of the elements present in a period also change.
(3) Atomic radii of the elements in a period decrease from left to the right.
(4) Along a period, the metallic character of the elements decreases and the non-metallic character increases.
(5) Along a period, the reducing character of the elements decreases and their oxidising character increases.
Advantages of the Modern Periodic Table
(1) The position of the elements in the periodic table are linked with their electronic configuration.
(2) Each group is an independent group and the idea of sub-groups has been discarded.
(3) One position for all the isotopes of an element is justified since the isotopes have the same atomic number. For the three isotopes of the element i.e., protium, deuterium and tritium have atomic number one.
(4) The positions of certain elements which were earlier misfits in the Mendeleev’s periodic table are now justified because it is based on atomic number of the elements.
(5) The division of the elements into s, p, d and f blocks has been quite helpful in under standing their properties.
(5) It is quite easy to remember and reproduce.
Mam your explanation is excellent so why are you not starting your youtube channel.
Mam i like your notes very much since they are very easy and ordered
Your notes are very well written. The exact amount of information needed. Thanks
Thank u so much ma’am very useful answers
Mam I like your notes very much, it is very easy to understand
very good explanation mam,very easy to understand.
Excellent explanation and very helpful. thank you very much.