Question 1 What are acids? Give example?
Question 2 What are organic acids. Give example?
Question 3 What are mineral acids. Give example?
Question 4 What are strong acids?
Question 5 What are weak acids?
Question 6 What are dilute acids?
Question 7 What are concentrated acids?
Question 8 Which gas is usually liberated when an acid react with a metal. How will you for the presence of this gas?
Question 9 While diluting an acid, why is it recommended that acid should be added to water and not water to the acid?
Question 10 Why should curd and other food stuffs not be kept in copper and brass vessels?
Question 11 What happens when an acid reacts with a metal oxide. Give equation of the reaction involved?
Question 12 What happens when an acid reacts with a metal carbonate. Give equation of the reaction involved?
Question 13 What happens when an acid reacts with a base?
Question 14 What is a neutralisation reaction. Explain with example?
Question 15 What happens when an acid reacts with a metal oxide?
Question 16 If someone is suffering from problem of acidity after overeating, which remedy would you suggest?
Question 17 What happen when carbon dioxide is passed through lime water?
Question 18 What happen when excess of carbon dioxide is passed through lime water?
Question 19 What happen when dilute hydrochloric acid reacts with magnesium ribbon?
Question 20 Which element is common in all acids?
Contents
Acids
Acids are those substances which are sour in taste. They change blue litmus to red.
For Example: The sour taste of lemon is due to the citric acid present in it. Fruits, curd are sour due to the presence of acids.
The acids present in plant materials and animals are called organic acids. For Example: Acetic acid (Vinegar), Citric acid (lemon, orange), Lactic acid (curd or milk), Tartaric Acid (tamarind), Formic acid etc.
The acids prepared from the minerals of the earth are called mineral acids. For Example : Sulphuric acid, Nitric acid, Hydrochloric acid etc.
Strong Acid
Sulphuric acid, Nitric acid, Hydrochloric acid etc.
Weak Acid
Acetic acid, Formic acid, Citric acid, Carbonic acid etc.
Concentrated Acid
It is one which contains the minimum possible amount of water.
Dilute Acid
It contains much more of water in it.
Diluting an Acid
The process of mixing the concentrated acid with water is highly exothermic. The dilution of concentrated acid should always be done by adding concentrated acid to water gradually with stirring and not by adding water to concentrated acid.
When concentrated acid is added to water for preparing a dilute acid, then heat is evolved gradually and easily absorbed by large amount of water.
If water is added to concentrated acid then large amount of heat is evolved at once. This heat changes some of the water to steam explosively which can splash the acid on our face or clothes
Properties of Acids
1) They are sour in taste
2) They turn blue litmus to red.
3) When acids are dissolved in water, they conduct electricity.
4) Acids react with metals to form hydrogen gas.
In order to detect hydrogen gas, bring a candle near the mouth of test tube. The gas will burn brightly with a pop sound. Curd and other sour substances should not be kept in metal vessels(like Cu or Brass) because they contain acids which react with metal of the vessel to form poisonous compounds which can damage our health.
5) Acids react with Metal carbonates and metal bicarbonates to form carbon dioxide gas.
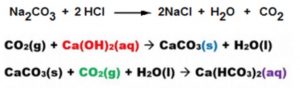
When Carbon Dioxide is passed through lime water, it turns milky. If excess of carbon dioxide is passed through the lime water, white precipitate dissolve and soluble compound calcium hydrogen carbonate is formed. If a person is suffering from the problem of acidity after overeating, we can suggest taking baking soda solution as remedy because baking soda react with excess of Hydrochloric acid in the stomach and neutralises it. This gives relief to the person.
Dilute sulphuric acid reacts with metal carbonates to form metal sulphates, carbon dioxide and water.
Limestone, marble, chalk are different forms of calcium carbonate.
6) Acids react with bases to form salt and water(Neutralisation reaction)
The reaction between acid and base to form salt and water are called as neutralisation reaction.
HCl + NaOH → NaCl + water
7) Acids react with metal oxide to form salt and water.

Acids also react with metal hydroxides to form salt and water. The antacid called Milk of Magnesia which is used to remove indigestion is a metal hydroxide called magnesium hydroxide. It is basic in nature.
8) Acids have corrosive nature
Mineral acids cause severe burns on the skin and attack and eat up materials like cloth, wood, stonework etc. They are never stored in metal containers because they corrode and eat up the metal container.
This was very helpful in making notes of chapter
Thanks a lot for your help
Just found out about this website this was really helpful. Thank you.
Thank u,it helps a lot
Very helpful thank you
Thank u it’s to useful
Very helpful for me ma’am