Question 1 What are isobars? Question 2 Give example? Isobars Isobars are the atoms of different elements having different atomic number but same mass number. For Example: … [Read more...] about Isobars
Structure of an atom
Isotopes
Question 1 What are isotopes? Question 2 Give examples of isotopes? Question 3 Give few application of isotopes? Question 4 why isotopes have same chemical properties? Question 5 Why isotopes have different physical properties? Isotopes Isotopes are atoms of same element having same atomic number but different mass numbers. For Ex: 1)Isotopes of … [Read more...] about Isotopes
Valency
Question 1 Define atomic number? Question 2 Define mass number? Question 3 Define valency? Question 4 What are valence electrons? Question 5 What are noble gases? Question 6 An element has atomic number 12.How many electrons are present in K,L,M shell? Question 7 Write the distribution of electrons in an atom of element whose atomic number is 18? Atomic … [Read more...] about Valency
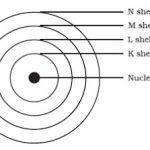
Arrangement of Electrons in the Atoms
Arrangement of Electrons in the Atoms 1) The energy levels of electrons are denoted by the numbers 1,2,3,4... and shells are represented by K,L,M,N..... 2) The arrangement of electrons in various energy levels or shells of an atom is known as electronic configuration of the element. The maximum number of electrons which can be accommodated in any energy level of an atom … [Read more...] about Arrangement of Electrons in the Atoms
Bohr’s Model of an Atom
Question 1 Describe Bohr's model of an atom? Question 2 How did Neil Bohr's explain the stability of atom? Question 3 Why is an atom neutral inspite of the presence of charged particle in it? Bohr's Model Of An Atom 1) An atom is made up of three particles: electrons, protons and neutrons. Electrons have negative charge, protons have positive charge whereas neutrons … [Read more...] about Bohr’s Model of an Atom