Question 1 Write the chemical name and formula of caustic soda?
Question 2 What happens when a concentrated solution of sodium hydroxide is electrolysed. Write the equation involved?
Question 3 Name the raw material used for the caustic soda?
Question 4 List the uses of caustic soda?
Question 5 What is a brine solution?
Sodium Hydroxide
It is commonly known as Caustic Soda. The formula of sodium hydroxide is NaOH.
Preparation
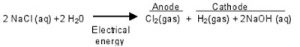
It is produced by electrolysis of a concentrated aqueous solution of sodium chloride(which is called brine solution)
When electricity is passed through a concentrated solution of sodium chloride, it decomposes to form sodium hydroxide, chlorine and hydrogen.
Uses of Sodium Hydroxide
(1) It is used for making soaps and detergents.
(2) It is used for making artificial textile fibres.
(3) It is used in the manufacture of paper.
(4) It is used in purifying bauxite ore.
(5) It is used in oil refining and making of dyes and bleaches.
Good
Thank you
Thanks
Nice
Thanks good nice
Thanks a lot
Thank u soo much
Thanks
Thank this website is great and helps a lot
Thanks
You are nice teacher who makes easy and useful notes thanks!!!!
good
very well explained content’ Thanks.
THANK U
I AM A MEMBER OF UR PREMIUM APP
Very nice explanation
Very helpful…..
thanks a lot.
Awsm