Question 1 Write the chemical formula and name of plaster of paris?
Question 2 What is gypsum?
Question 3 Name the calcium compound which hardens on wetting with water?
Question 4 What will happen if heating is not controlled while preparing plaster of paris?
Question 5 Name the compound which is used in hospitals for setting fracture bones?
Question 6 How is plaster of paris prepared from gypsum?
Question 7 Give few uses of plaster of paris?
Contents
Plaster of Paris
Plaster of paris is calcium sulphate hemihydrate.
The chemical formula of Plaster of paris is CaSO4·½H2O
It is known as P.O.P.
Preparation of Plaster of Paris
It is prepared from gypsum.
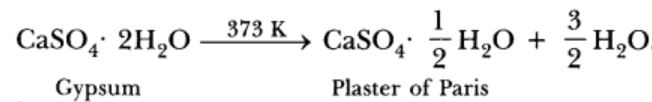
Gypsum is calcium sulphate dihydrate. The chemical formula of gypsum is CaSO4·2H2O
Plaster of Paris is prepared by heating gypsum to a temperature of 373K.When gypsum is heated to a temperature of 373k ,It loses three-Fourths of its water of crystallisation and forms Plaster of Paris.
Properties of Plaster of Paris
(1) It is a white powder.
(2) It has a very remarkable property of setting into a hard mass on wetting with water.
P.O.P. should be stored in a moisture proof container.
Uses of Plaster of Paris
(1) It is used in hospitals for setting fractured bones in the right position to ensure correct healing.
(2) It is used in making toys, decorative materials, cheap ornaments, cosmetics, black-board and casts for statues.
(3) It is used in chemistry laboratories for sealing air gaps in apparatus where air-tight arrangement is required.
(4) It is used for making surfaces smooth before painting.
It is good
Osm notes
Great work!!
Great work!!
Loved it!!
Informative !!!!!!
Nice notes
Very good I like it
Bhahut ache notes hai
Nice notes
Awesome
Very much informative
Yey good work man
Simply and briefly answered
Thanks for the help
Nice notes
Never seen like this
Now I can learn easily from yours note thanks for this
Worth one.
Appreciable and helping one
I understand very easily.
I like it.
Thankyou! For giving me best notes…… thanks a lot!!
You are just amazing
nice explanation makes difficult easy
Very easy way
They thought us in very easy ways
Awesome notes
thankyou so much for this brief notes. i got full marks by studying this
Very helpful…….. I understood everything earlier this topic was tough for me now it’s easy.
It is really helpful
Nice definition, able to understand easily,
Nice , very nice notes,
Mam can you be my teacher of chemistry
Very nice..
Very useful..
Point wise ..
Easily understood..
It helps in my holiday homework
Thanks…..,
And it was very helpful for learning all main points were described…..,
Again thak you very much…….,