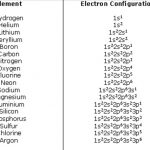
The distribution of electrons into different shells, sub shells and orbitals of an atom is called its electronic configuration. The electronic configuration of any orbital can be represented as: nlx n is the number of principal shell, l = symbol of the sub shell or orbital, x= number of electrons present in the orbital 4p1 means that p- sub shell of the 4th main shell … [Read more...] about Electronic Configuration of Elements
Structure of Atom
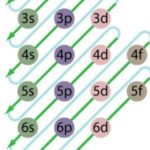
Energy Level Diagram
Energies of orbitals of hydrogen and hydrogen like particles depend upon the value of principal quantum (n) number only , those of multi-electron atoms depend both upon principal quantum number ( n ) as well as azimuthal quantum number(l). Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams. Important … [Read more...] about Energy Level Diagram
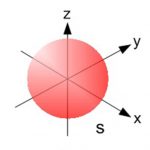
Shapes of Atomic Orbital
An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum. The probability at any point around the nucleus is calculated using schrodinger wave equation and is represented by the density of the points. Shape of s orbital For the coordinates( x, y, z) of the electron with respect to the nucleus, … [Read more...] about Shapes of Atomic Orbital
Pauli Exclusion Principle
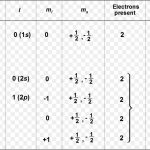
Wolfgang Pauli a German physicist in 1925 put forward a principle known after his name as Pauli exclusion principle. No two electrons in an atom can have the same set of four quantum number. In an atom, any two electrons may have the same values for any of the three quantum numbers but the 4th must be different. Any particular orbital is described by three quantum … [Read more...] about Pauli Exclusion Principle
Quantum Numbers
Quantum Numbers An atom contains a large number of orbitals. These are distinguished from each other on the basis of their shape, size and orientation in space. These characteristics of an orbital are expressed in terms of three numbers, called principal, azimuthal and magnetic quantum number. Quantum numbers may be defined as a set of 4 numbers with the help of which we … [Read more...] about Quantum Numbers