Question 1 Name one metal which has a low melting point? Question 2 Name two metals which are malleable and ductile? Question 3 Name one soft metal that can be cut with knife? Question 4 Name the metal which is the poorest conductor of heat? Question 5 Why copper and aluminium metals are used for making electric wires? Question 6 Why copper and aluminium metals … [Read more...] about Metals
Metals and Non-metals
Reaction of Metal with Chlorine and Hydrogen
Question 1 What happens when metals react with chlorine? Question 2 What happens when metals react with hydrogen? The reaction of metal with Chlorine Metals react with chlorine to form ionic chlorides. 2Na(s) + Cl2 (g) → 2 NaCl(s) Ca (s) + Cl2 (g) → CaCl2 Mg (s) + Cl2 (g) → MgCl2 The reaction of Metals with Hydrogen Most of the metals do not combine with … [Read more...] about Reaction of Metal with Chlorine and Hydrogen
Alloys
Question 1 What is an alloy? Question 2 Give two examples of alloys? Question 3 How are the properties of an alloy different from those of the constituent metals? Question 4 What elements are present in steel? Question 5 What are the constituents of stainless steel? Question 6 State an alloy of copper? Question 7 Give the constituent of brass? Question 8 … [Read more...] about Alloys
Corrosion
Question 1 Define the term corrosion? Question 2 What is meant by rusting of iron? Question 3 State the conditions necessary for rusting of iron? Question 4 State the ways to prevent the rusting of iron? Question 5 Name the metal used for galvanising iron? Question 6 Why copper objects lose their shine after sometime? Question 7 Why silver articles became … [Read more...] about Corrosion
Refining
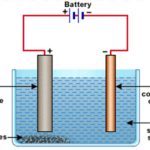
Question 1 What is meant by refining of metals? Question 2 Define the term electrolytic refining? Question 3 How will you refine copper by electrolytic refining? Refining The process of purifying impure metal is called refining of metal. The most important and most widely used method for refining impure metals is called electrolytic refining. Many metals like … [Read more...] about Refining